
- Posted
- Categories
-
- Research Integrity
Research Integrity Update - Feb 2024
Details on two new papers from the Bennett Institute Research Integrity Team.
Latest news and views from around the Bennett Institute

Details on two new papers from the Bennett Institute Research Integrity Team.
Here we describe some of our recent work investigating the impact of the COVID-19 pandemic on safe prescribing using a set of quality assured indicators across 57 million patients’ records in England

In the second of a two-part blog we explain why OpenPrescribing figures don’t always match with other prescribing data resources, due to differences in how data is presented.

In the first of a two-part blog we explain why OpenPrescribing figures don’t always match with other prescribing data resources, due to differences in the data source.

In this guest blog, Mark Green describes their latest paper using OpenSAFELY.
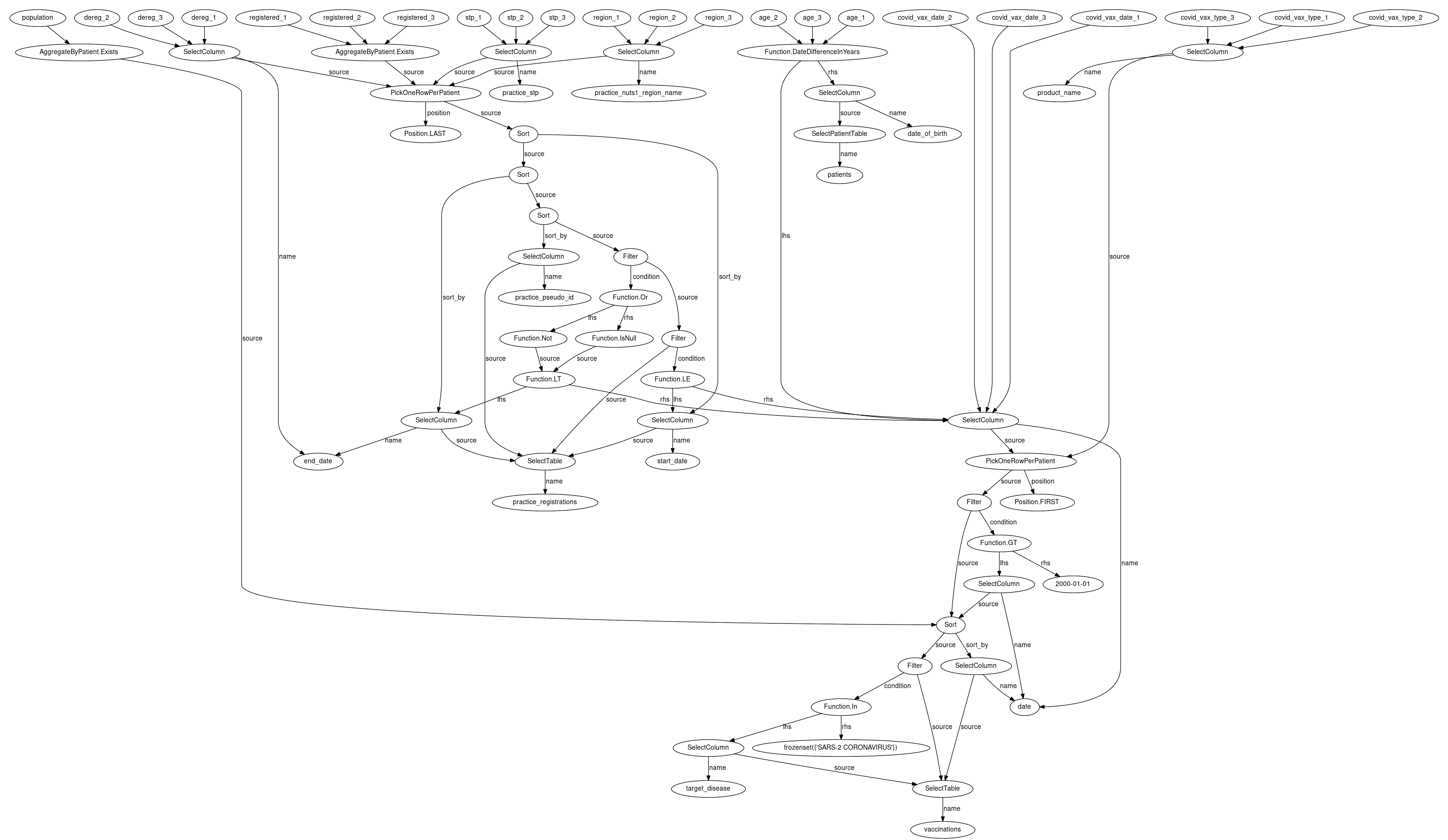
How can you be confident that the queries you write will be correctly interpreted and return the expected results?

To accompany the recent Ethnicity Short Data Report preprint we have created a short explainer for researchers using ethnicity in OpenSAFELY-TPP

We have just advertised an exciting and unusual new job. An exciting fellowship based at Jesus College, and working with our amazing team. Closing date: 09/02/2024 12:00 pm.

This blog describes how we develop new measures at OpenPrescribing.

The final session was a series of talks highlighting some of the research conducted in OpenSAFELY, some external perspectives on OpenSAFELY, and concluding remarks from our benefactor Peter Bennett, and director Ben Goldacre.

The third session in the Bennett Conference was a series of talks describing the overall operation of the OpenSAFELY platform and service.

The second session in the Bennett Conference was a series of talks covering the history, technical underpinnings, research and use cases of OpenPrescribing.

The first session in the Bennett Conference discussed past and present work of the Institute on research integrity and policy issues.

A whistlestop tour of the whole history of our group, from OpenPrescribing via TrialsTracker to OpenSAFELY and on through Open Science, policy work, and more

The COVID-19 pandemic has reshaped many aspects of healthcare, including how antibiotics are prescribed in primary care settings. In this guest blog, Professor Diane Ashiru-Oredope lead pharmacist for antimicrobial resistance at UK Health Security Agency and our own Brian MacKenna, share some insights on repeat antibiotic prescribing and the link to health inequalities from a recent UK Health Security Agency analysis using OpenSAFELY.
A common question asked of the Bennett Institute is ‘have you got a tool to map BNF codes to dm+d codes?’ We’ve created one to download

We’ve been busy creating new measures for OpenPrescribing.net, and reviewing ones which aren’t needed any more.

We’ve been busy doing housekeeping on OpenPrescribing.net measures. Here’s a summary of the main changes.