- Posted
- Categories
-
- OpenPrescribing
Your Organisation's OpenPrescribing Custom Email Alerts
At OpenPrescribing we know that clinicians can be overwhelmed with guidance and data about many different aspects of care. We therefore have developed an innovative email alert service for every single practice, primary care network and clinical commissioning group in England that delivers bespoke custom emails to your inbox about your own organisations prescribing.
To sign up to your organisation’s alerts all you need to do is:
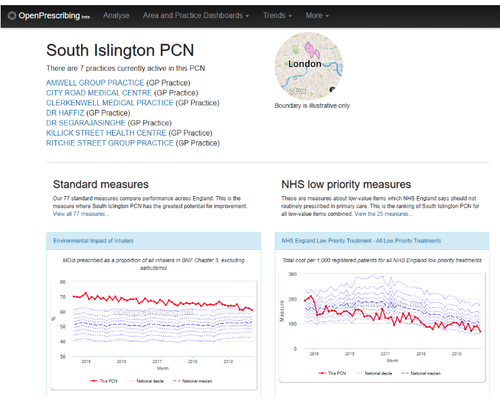
- Go your organisation’s homepage. Search here for your:
- Enter your email in the subscribe box at the bottom of the dashboard (see picture below)
- HIT SUBSCRIBE
These email alerts help people keep track of their prescribing trends, alert you to changes in prescribing that may be difficult to spot and importantly tell you if your prescribing has changed in comparison to your peers. You can see an example of an OpenPrescribing practice email alert here and you can read more about the methods in our paper.