Classifying and measuring medicines usage with the ATC/DDD system
- Posted:
- Written by:
- Categories:
This article is part of a series: OpenPrescribing Hospitals: Categorising Medicines
- Classifying and measuring medicines usage with the ATC/DDD system
The ATC/DDD system
The ATC/DDD system is a way for classifying and measuring drug utilisation to enable comparisons of drug use between countries, regions, and other healthcare settings. It classifies medicinal products using the Anatomical Therapeutic Chemical (ATC) classification and provides the Defined Daily Dose (DDD) as a way to measure their usage.
It is maintained by the World Health Organisation (WHO) Collaborating Centre for Drug Statistics Methodology and is widely used internationally.
Anatomical Therapeutic Chemical classification system
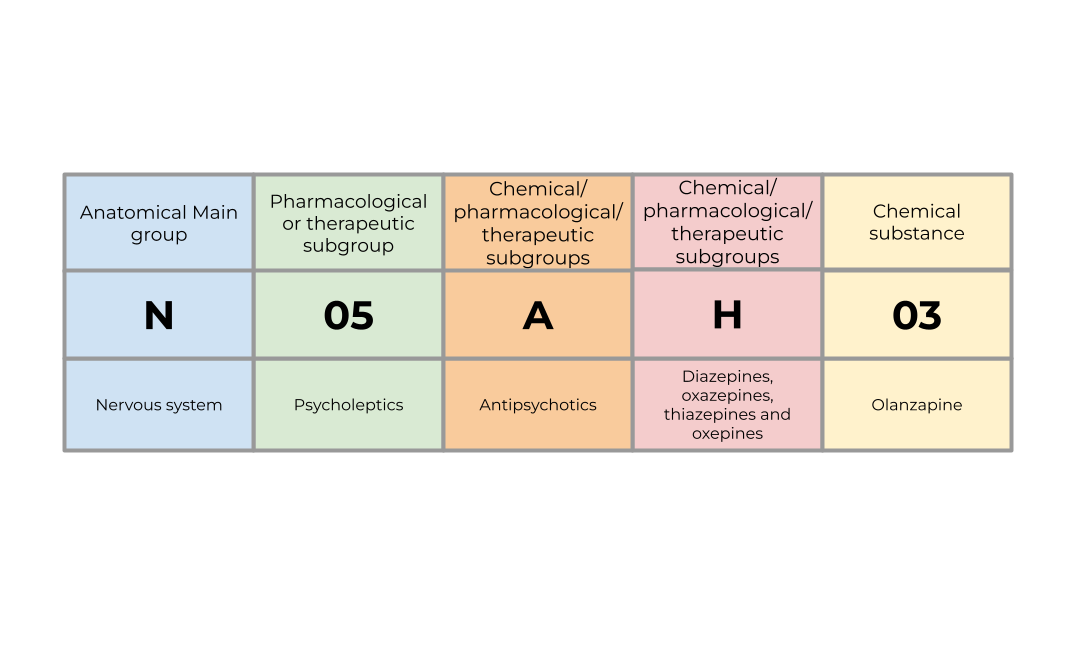
The ATC classification system provides a classification for the active ingredients within medicinal substances based on where in the body they act and their therapeutic (what condition or disease they treat), pharmacological (how they work in the body), and chemical properties (the structure and composition of the substance). The system is a hierarchy with five levels:
-
Anatomical main group
- Identifies the organ or system in the body that the drug primarily targets.
- Represented by a single letter
- E.g.
Nrepresents the nervous system
-
Pharmacological or therapeutic subgroup
- Classifies drugs based on either:
- Their therapeutic use
- Their pharmacological effect
- Represented by two digits
- E.g
N05represents psycholeptics.
- Classifies drugs based on either:
3/4. Chemical/pharmacological/therapeutic subgroups
- Further refines the classification based on:
- Chemical properties
- Pharmacological effect
- Therapeutic category
- Each represented by a single letter
- E.g.
N05Arepresents antipsychotics,N05AHrepresents diazepines, oxazepines, thiazepines and oxepines.
- Chemical substance
- Identifies the specific chemical compound for the active ingredient
- Represented by two digits
- E.g
N05AH03represents olanzapine

Principles for assigning ATC codes
The ATC system classifies medicinal products according to their main therapeutic use. Each medicinal product should have a single ATC code indicating it’s main therapeutic use, where a medicinal product is defined by:
- Active ingredients
- Route of administration
- Strength
This means that:
- Formulations with the same ingredients, route of administration and strength share the same ATC code, but an ATC group may include medicinal substances with various indications
- Medicinal substances with different strengths or routes of administration, for different therapeutic uses, may be given different codes, even if they contain the same active ingredient. For example, the corticosteroid prednisolone has multiple uses with different routes of administration, so has multiple ATC codes:

Challenges with assigning ATC codes
The principles above support the classification of a wide range of medicinal substances, but there are some cases where classification can be challenging.
Products with multiple therapeutic uses
Sometimes, a single medicinal product (same active ingredient, strength and route of administration), can have two or more equally important therapeutic uses. Some countries may use the substance for one indication, and other countries may use it for another.
In these cases, where there may be more than one classification option, the WHO decides a main indication. An example is metronidazole, which is used as an antibacterial and antiprotozoal (administered orally, with the same strength). In the ATC system, oral metronidazole is classified under antiprotozoals (P01), not under antibacterials for systemic use (J01), which the parenteral formulations of metronidazole are classified under.
Combination products
Some medicinal products contain two or more active ingredients. These are known as combination products. In the ATC system, these products are given separate ATC codes from products containing only one of the active ingredients, but are classified using the same principles. An example is formoterol and beclometasone inhalers (R03AK08), which are classified separately to beclometasone inhalers (R03BA01) and formoterol inhalers (R03AC13).
Defined Daily Dose
The ATC system provides a way to classify drugs, but not a unit to measure them in. This is what the Defined Daily Dose (DDD) is for. DDDs are a unit of measure for medicines consumption that enables comparison of usage across groups of medicines. They are defined by the WHO as:
Defined Daily Dose (DDD): The assumed average maintenance dose per day for a drug used for its main indication in adults
DDDs are only assigned to drugs with an ATC code (though not all ATC codes have associated DDDs) and only one DDD is assigned per ATC code and route of administration (there are a small number of exceptions to this rule, such as erythromycin, which has two DDDs for oral formulations, to be picked based on what salt they contain).
The usage of a drug in DDDs can be calculated as follows:

Here’s an example for prednisolone tablets:

Accessing the ATC/DDD
The ATC/DDD index is maintained by the WHO. They provide a searchable version of it here and there is also a purchasable machine-readable version, released annually.
The ATC/DDD index is continually updated, with additions and alterations published alongside the main index.
Further information
More information about the ATC system and DDDs can be found in these guidelines published by the WHO. We’re already using the ATC/DDD system in OpenPrescribing Hospitals to group medicines and measure their usage for the Measures feature. We’ll also be exposing DDDs in other parts of the platform, which we’ll write more about soon.
If you have any questions, or more insight into the ATC/DDD system that you can share please get in touch at bennett@phc.ox.ac.uk.


