Calculating Defined Daily Dose quantity in the Secondary Care Medicines Data
- Posted:
- Written by:
- Categories:
This article is part of a series: OpenPrescribing Hospitals: Measuring Quantity
- Measuring quantity in the Secondary Care Medicines Data
- Calculating unit dose quantity in the Secondary Care Medicines Data
- Calculating ingredient quantity in the Secondary Care Medicines Data
- Calculating Defined Daily Dose quantity in the Secondary Care Medicines Data
- OpenPrescribing Hospitals Product Lookup
In the previous posts in this series, we’ve described how quantity is measured in the Secondary Care Medicines Data (SCMD) and how additional product information in the dictionary of medicines and devices (dm+d) format can be used to calculate unit dose quantity and ingredient quantity. The dm+d is not the only coding system that allows identification and measurement of medicines. This post will look at another system, the ATC/DDD system, and how it can be used to calculate Defined Daily Dose quantity, the final measure of quantity we will be using on OpenPrescribing Hospitals.
What is a Defined Daily Dose?
A Defined Daily Dose (DDD), is a unit of measure for drug utilisation. It forms part of the ATC/DDD system for classifying and measuring drug usage. We’ve written a detailed post explaining the ATC/DDD system which we recommend reading first, but below is a short summary.
The ATC/DDD system is a way for classifying (using the ATC) and measuring (using DDDs) drug utilisation, maintained and published by the World Health Organisation Collaborating Centre for Drug Statistics Methodology.
The Anatomical Therapeutic Chemical (ATC) system is a hierarchical coding system used to classify active ingredients within medical substances based on where in the body they act, and their therapeutic, pharmacological, and chemical properties.
A DDD is a standardised unit of drug utilisation that allows comparison of drug use between different countries, regions and healthcare settings. DDD values are assigned to ATC codes and they are defined by the WHO as:
Defined Daily Dose (DDD): The assumed average maintenance dose per day for a drug used for its main indication in adults
By mapping the product codes used within the SCMD to ATC codes, we can find the DDD for individual products and where applicable, calculate the quantity of a medication issued in DDDs.
Why are DDDs useful for OpenPrescribing Hospitals?
In the previous post in this series, we introduced ingredient quantity as a way of measuring medicines usage across multiple preparations with varying amounts of active ingredients. This is useful when you’re interested in comparing multiple products with the same ingredient (such as all paracetamol preparations), but is not always appropriate if you want to compare usage across products with different ingredients.
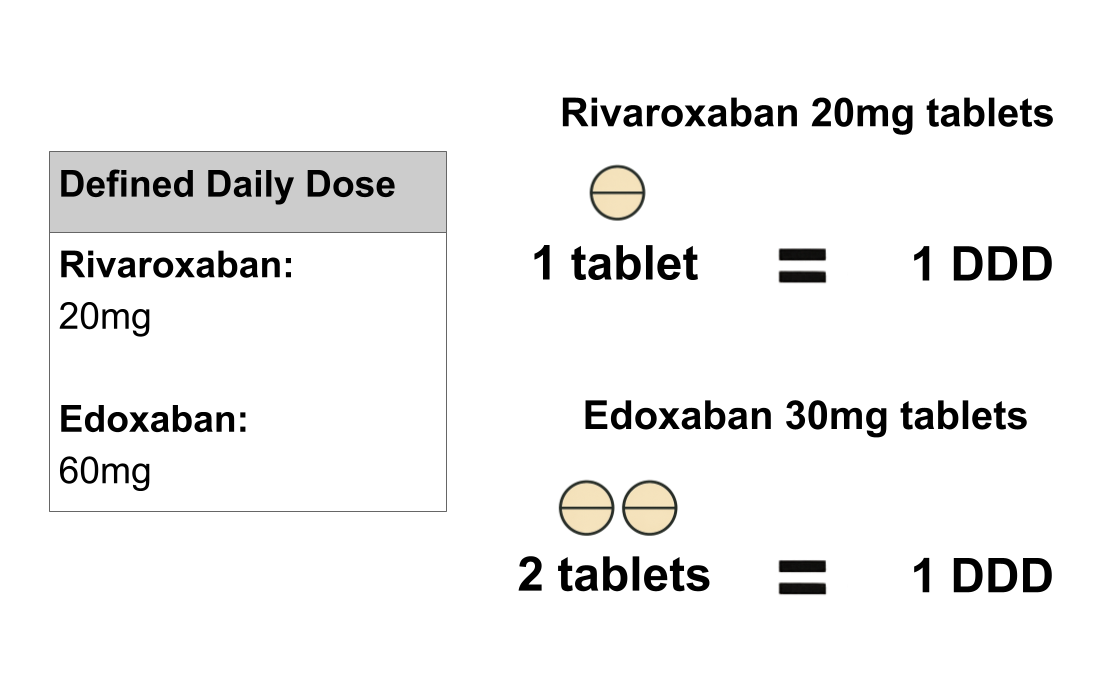
Take the case of direct oral anticoagulants (DOACs), examples of which are shown in the table below. There are several available DOACs, which despite having the same mechanism of action and indication for use, have different doses for the same indication; 10mg of apixaban is not equivalent to 10mg of edoxaban.
| DOAC | Product name | Ingredient | Strength | DDD |
|---|---|---|---|---|
| Apixaban | Apixaban 2.5mg tablets | Apixaban | 2.5mg | 10mg |
| Rivaroxaban | Rivaroxaban 10mg tablets | Rivaroxaban | 10mg | 20mg |
| Edoxaban | Edoxaban 30mg tablets | Edoxaban tosilate | 30mg | 60mg |
| Dabigatran | Dabigatran 75mg capsules | Dabigatran etexilate mesilate | 75mg | 0.3g |
To compare the usage of these products, we need a measure of quantity that takes into account the amount of each drug expected to be used to have its desired effect. This is what DDDs can be used for (for an actual example, see our measure looking at issuing of best value DOACs).
Calculating DDD quantity
Step 1: linking the ATC/DDD to the SCMD
The ATC code for individual products is not available within the SCMD (see this post for a reminder of what is available). Fortunately, this mapping has been done by the team that maintains the dictionary of medicines and devices (dm+d), and is made available as a supplementary file published alongside the dm+d with each weekly release.
Using this mapping, 11,146 (88%) of individual products within the SCMD can be linked to an ATC code. The supplementary dm+d file also contains the mapping of ATC codes to DDD values, but we use the mapping published by the WHO, which we know to be up to date. Of the products with an ATC code, 5,762 (52%) have an associated DDD value in the WHO ATC/DDD index.

For products with an associated ATC and DDD value, the next step is to check whether the information contained within the dm+d for those products is compatible with the DDD values (which are assigned for specific routes of administration, with units of measure that may not easily convert to those used in the SCMD).
Step 2: Check DDD route of administration compatibility
As DDDs are specified for specific routes of administration, we need to check that the routes of administration associated with DDD values are aligned with the routes of administration for a product in the dm+d.
The WHO ATC/DDD index and dm+d use different systems for representing the route of administration, so the first step is to create a mapping between these two systems. We convert the routes of administration used in the dm+d to those used by the WHO. We won’t go into the details of this here, but you can see the method here.
The majority (99.6%) of products in the SCMD with a linked DDD value have a route of administration that maps to a WHO route of administration, which you can see in the flowchart below.

For a small number of products, the routes of administration are aligned, but the product has multiple DDD values, determined by factors such as chemical salt or drug formulation. For example, amphotericin has multiple DDDs associated with the same route of administration. For many of these cases, there are comments provided alongside the DDD values indicating when each should be used. We’ll need to look at these comments and products they relate to in more detail before making a choice on which DDD is most appropriate.
Step 2: Check DDD unit compatibility
The next step is to check the compatibility between the units of measure used in the SCMD and the units of measure matched DDDs are measured in.
A small number of products (<1%) are reported in the SCMD using the same units as their corresponding DDDs. For these the DDD quantity can be calculated by dividing the SCMD quantity by the DDD value. An example is paromomycin 250mg capsules, which have a DDD of 3g and are reported in the SCMD in grams.

For those that are not, we have to instead use the ingredient quantity. This can only be done for products with:
- A single active ingredient (products with more than 1 active ingredient are regarded as combinations in the ATC system and are given a separate ATC code. Calculation of DDDs for these products is a bit more complicated, so calculating DDD quantity for them is something we’ll come back to)
- A calculated ingredient quantity (see the previous post for what this requires)
- Ingredient quantity units compatible with DDD units
Where these conditions are met, the DDD quantity is calculated by dividing the ingredient quantity by the DDD value. An example is shown below. Nicotine chewing gum has a single ingredient (nicotine), which is measured in mg, as is the DDD value for drugs used in nicotine dependence, formualated as chewing gum.

How many products can DDD quantity be calculated for?
The number of products reported in the SCMD we can calculate DDD quantity for is shown in the flowchart below. Overall, DDD quantity can be calculated for around 40% of products reported in the SCMD. This is lower than other measures of quantity becuase a large number of ATC codes have no assigned DDD values. For products linked to ATC codes which do have an assigned DDD value, DDD quantity can be calculated for 95%.

DDD quantity is the final measure of quantity that we will be using on the OpenPrescribing Hospitals platform. We think it will be one of the most useful. Next up, we’ll show the Product Lookup feature on OpenPrescribing Hospitals, that allows you to see all of the available quantity information discussed in this series for every product reported in the SCMD.


