Calculating unit dose quantity in the Secondary Care Medicines Data
- Posted:
- Written by:
- Categories:
This article is part of a series: OpenPrescribing Hospitals: Measuring Quantity
- Measuring quantity in the Secondary Care Medicines Data
- Calculating unit dose quantity in the Secondary Care Medicines Data
- Calculating ingredient quantity in the Secondary Care Medicines Data
- Calculating Defined Daily Dose quantity in the Secondary Care Medicines Data
- OpenPrescribing Hospitals Product Lookup
In the previous post we detailed the processing steps required to generate a reliable measure of quantity using the Secondary Care Medicines Data (SCMD). Using supplementary information available in the dm+d, we can calculate another quantity measure, Unit dose quantity, which will be the topic for this post. If you’re not already familiar with the dm+d, we recommend first reading our dm+d explainer. We’ve also added a glossary at the end to help reference the technical terms and acronyms used throughout this post.
What are unit doses?
A unit dose is a single measured quantity of a medicine, packaged for administration to a patient at one time. For example, a tablet or a vial to be injected. This is different to dose, which is the quantity of medication given as part of a broader dosage regimen that includes frequency and duration. Below are a couple of examples to highlight this difference.
Not all products can be packaged in well defined units, such as tablets, which are defined as having a discrete dose form. Products such as creams and gels are most commonly packaged in tubes, from which it is harder to administer fixed quantities. Such products are said to have a continuous dose form. For other products, such as colostomy bags — medical devices used for collection rather than delivering medicine — the concept of a dose doesn’t make sense. Unit doses are therefore only defined for products with a discrete dose form.
| Dose form | Definition |
| Discrete | A dose form where the medicine is packaged in well-defined, individual units that can be counted and administered as single measured quantities. These products have clear unit doses that can be precisely measured and administered. Examples include tablets, capsules, pre-filled syringes, and vials for injection. |
| Continuous | A dose form where the medicine cannot be naturally divided into discrete units. These products are packaged in containers from which variable amounts can be administered, making it more challenging to ensure consistent quantities. Examples include creams, gels, ointments, and liquid medications that need to be measured out for each dose. |
| Not applicable | A category for products where the concept of a dose is not relevant or meaningful. These are typically medical devices or products that are used for collection, access, or other purposes rather than delivering medication. Examples include colostomy bags and catheters. |
The dose form and associated unit dose information for individual products can can be found in the dm+d. We’ll have a look at the extra information available below.
Unit dose information
Unit doses are specified in several fields within the dm+d, shown in the figure below. These include the unit of measure for the unit dose and the size of the unit dose, which has a separate unit of measure. The unit of measure for the unit dose may not always be the same as the unit of measure of the unit dose itself. For example, a liquid medicine can have a unit dose measured in vials, with each vial being 2 ml.

Unit doses are a familiar unit of measure
We want to express the quantities of medicines issued in units of measure that are most familiar to those involved with issuing and administering them. This is not always the unit of measure that is reported in the SCMD. A good example is a pre-filled injection syringe. By being packaged with a medication or solution already loaded into a sterile, disposable syringe, they simplify the process of administering injectable medications. When measuring quantities of these products, you may therefore want to count the number of individual syringes.
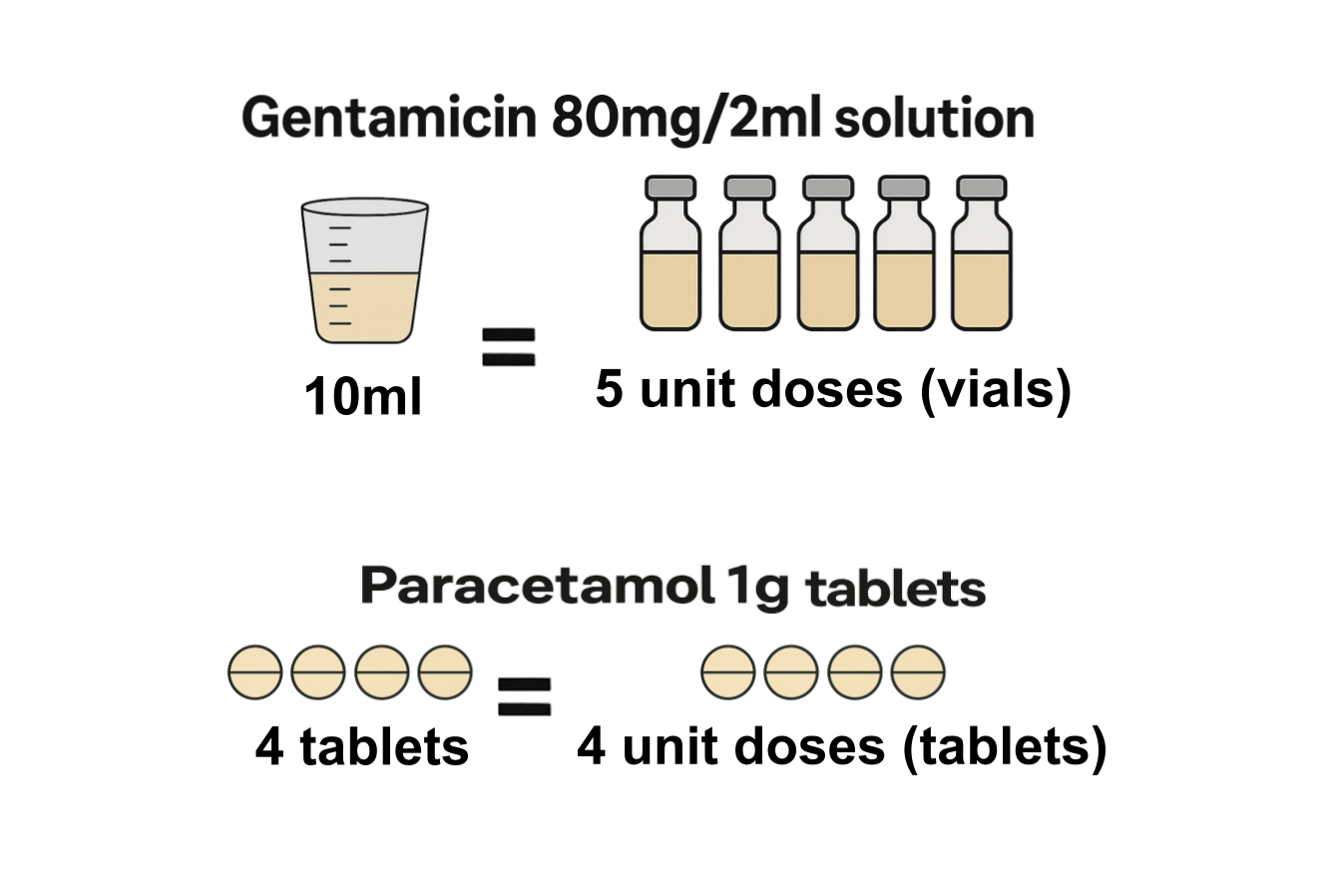
However, in the SCMD, many of these products are reported in ml. Below we show how this can be converted into unit doses, which for this product, is the number of syringes.
Calculating quantity in unit doses
The quantity in unit doses is calculated using the extra information from the dm+d described above. To calculate the quantity in unit doses, we have to check:
- Does the product have a discrete dose form?
- Are the units of measure for the product in the SCMD compatible with the unit dose information in the dm+d?
The flowchart below shows the number of individual products reported in the SCMD for which the quantity in unit doses can be calculated. Of the 12,733 products, 7,816 (61%) have a discrete dose form.

For those with a discrete dose form, we then check if the unit of measure for the SCMD quantity is the same as the unit dose form size (UDFS) unit of measure. For example, the UDFS for Paracetamol 100mg/10ml solution for infusion ampoules is 10ml, so we check that the SCMD quantity is also in ml, which it is. If instead, it was reported in grams, calculation of unit dose quantity would be more challenging. Fortunately, all of the UDFS units and SCMD units for products with discrete dose forms line up.
Following this check, the quantity in unit doses can then be calculated by dividing the SCMD quantity by the UDFS. As an example, 6ml of Tinzaparin sodium 12,000 units/0.6ml can alternatively be expressed in unit doses (measured in pre-filled syringes) by dividing the SCMD quantity (6ml) by the UDFS (0.6ml), giving 10 pre-filled syringes. This is a simpler unit of measure to deal with for this product.

The quantity in unit doses is an alternative measure of quantity that can be calculated for a large proportion of individual products which can express usage in more familiar units. In the next post we will describe how we can calculate the quantity of each ingredient within a product, the Ingredient quantity.


