Calculating ingredient quantity in the Secondary Care Medicines Data
- Posted:
- Written by:
- Categories:
This article is part of a series: OpenPrescribing Hospitals: Measuring Quantity
- Measuring quantity in the Secondary Care Medicines Data
- Calculating unit dose quantity in the Secondary Care Medicines Data
- Calculating ingredient quantity in the Secondary Care Medicines Data
- Calculating Defined Daily Dose quantity in the Secondary Care Medicines Data
- OpenPrescribing Hospitals Product Lookup
In the previous post we described how we can use additional information from the dm+d to convert the SCMD quantity into the quantity in unit doses. In this blog we will describe how we can calculate a different measure of quantity, ingredient quantity.
Ingredient quantity is the amount of each active ingredient within a product
Medicines contain a wide range of ingredients. These ingredients can be of two types: active or inactive. Active ingredients are the ingredients within a product that produce the intended therapeutic effect, such as relieving pain. Inactive ingredients (or excipients) are ingredients that by themselves don’t provide any therapeutic effect, but help with the manufacturing, stability, appearance or palatability of a product.
Measuring the amount of each active ingredient within a product, the ingredient quantity, is an alternative way to measure medicines usage.
Calculating ingredient quantity is more complicated than calculating the quantity in unit doses for several reasons:
- It requires identification of the ingredients within a product (there can be many!)
- The amount (strength) of each ingredient has to be determined
- Ingredient strengths are reported in a wide range of units of measure that aren’t always compatible with the units used in the SCMD
Measuring ingredient strength
Ingredient information for individual products reported in the SCMD is contained within the dictionary of medicines and devices (dm+d) in the Virtual Product Ingredient (VPI) class. This class conains the following fields:
- Ingredient
- Strength value numerator
- Strength value numerator unit
- Strength value denominator
- Strength value denominator unit
Not all ingredients have strength information. For those that do, it can be given as a single value (the ingredient strength numerator), or two values (the ingredient strength numerator and denominator) to represent strength ‘per’ for ingredients such as solutions where there may be, for example, 10mg dissolved in 1ml of solution.
Below is an example for a product with a single ingredient. This product contains 250mg of paracetamol suspended in 5ml of the liquid. The strength is given as 50mg of paracetamol per ml of liquid, equivalent to 250mg per 5ml of the liquid.

Why is ingredient quantity useful?
To see why calculating ingredient quantity is useful, let’s look at how a selection of paracetamol products are recorded within the SCMD.
| Product name | Unit of measure | Unit dose unit of measure | Ingredient quantity unit |
| Paracetamol 500mg capsules | capsule | capsule | gram |
| Paracetamol 500mg tablets | tablet | tablet | gram |
| Paracetamol 500mg/5ml oral suspension | ml | N/A | gram |
| Paracetamol 500mg/50ml infusion bags | ml | bag | gram |
These four products are measured in three different units of measure in the raw data. Tablets and capsules of the same strength are comparable, but it’s more difficult to aggregate the quantities between capsules and ml. Unit dose quantity doesn’t help here; the oral suspension doesn’t have a unit dose, and whilst tablets are comparable with capsules, they are not with bags.
One thing that is common across all of these products is that they all contain the same single ingredient, reported in the same unit of measure. Calculation of ingredient quantity provides an alternative measure of quantity when aggregating across multiple products.
Calculating ingredient quantity
Step 1: identify the ingredients
Many products have a single ingredient, but others have a wide range of ingredients of varying strengths (e.g. infusion bags, which are bags of fluid containing multiple ingredients) and some products have no ingredients (e.g devices such as wound dressings and nitrile gloves) .
Ingredient quantity can only be calculated for products with specified ingredients, so the first step is to identify whether a product has any ingredients. 10,459 (82%) of products reported in the SCMD have at least one ingredient. In total there are 15,280 different product-ingredient combinations, for which we can try and calculate ingredient quantity. Products with multiple ingredients have multiple product-ingredient pairs. For example, co-codamol tablets contain two ingredients: paracetamol and codeine phosphate.
Step 2: identify the ingredient strength information
The next requirement is that there is strength information available for the product ingredients in the dm+d. Only a small number, 586 (4%), do not have this information (a large proportion of these products are vaccines or skin prick tests). For those than do, the next step is to see if the strength information is reported in units that are compatible with the units used to report quantity in the SCMD.

Step 3: check compatiblity of ingredient strength with the SCMD quantity unit
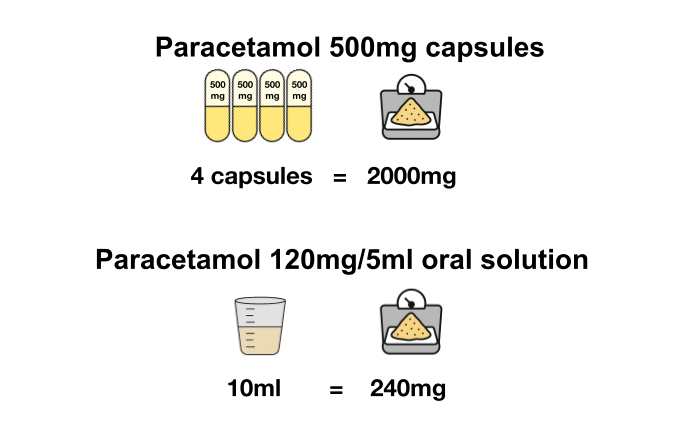
How you check compatibility of the ingredient strength depends on whether the strength is given as a single value (just the numerator) or with both a numerator and denominator as ‘per’ strengths. Where the strength is given as a single value, the ingredient quantity can be calculated by multiplying the SCMD quantity by the ingredient strength numerator. Below is an example.

Where the ingredient strength has a denominator component, it is more complicated. In these cases, we need to check whether the units of measure for the denominator are the same as the SCMD quantity units. For the product below, both the SCMD quantity and strength denominator are measured in ml, so we can get the ingredient quantity by multiplying the SCMD quantity by the strength numerator divided by the strength denominator.

If these units are not compatible, we can’t calculate the ingredient quantity. An example is given below.

For this product, the SCMD quantity is reported as the number of plasters. This is not the same as the strength denominator, which is reported in grams. Whilst it is obvious from the product name that each plaster contains 700mg of lidocaine, you can’t tell this from the structured information in the dm+d; there is 50mg of lidocaine per gram, but we don’t know how many grams make up a plaster. To be sure, we have to find the Actual Medicinal Products associated with this Virtual Medicinal Product and then look up the manufacturer’s information.
Each 10 cm x 14 cm plaster contains 700 mg lidocaine (equivalent to 5%w/w)
This confirms that each plaster contains 700mg of lidocaine and therefore, so we can work back and determine that each plaster must be 14g, which allows us to calculate the ingredient quantity as before. Let’s look at one more example.

Within the SCMD, this product is reported as the number of applicators issued. However, the strength information is given as the number of mg per g of cream. It is not clear, from the product name, or its strength information, how much cream is in a single applicator. Again, you can find this out if you look up the manufacturer’s information.
Each single use container contains 300 micrograms of alprostadil in 100 mg of cream.
Once we know this, we can calculate that 10 applicators contain 3000 micrograms of alprostadil.
How many product-ingredient combinations can ingredient quantity be calculated for?
The number of product-ingredient combinations we can calculate ingredient quantity for is shown in the flowchart below.

Of those with strength information, only 3% can’t be calculated due to incompatible units, but as shown above, this could be solved by looking at the manufacturer information for these products.
Ingredient quantity is an alternative measure of quantity that is particularly useful for comparing quantities across multiple products. In the next blog we’ll describe a related quantity measure, Defined Daily Doses, which are widely used for comparing usage of different types of medicines.


