This article is part of a series: Guest Blogs
- High Dose Dexamethasone
- How I use OpenPrescribing in my practice as a GP
- Conducting Research Using OpenSAFELY: My Experience of the Co-pilot Service
- Using electronic health records and open science in the COVID-19 pandemic
- Exploring the Impact of COVID-19 on common infections: Treatment Pathways, Antibiotic Prescribing, and Exposure
- Incidence and management of inflammatory arthritis in England before and during the COVID-19 pandemic
- Updates of OpenSAFELY Research on COVID-19 Therapeutics
- Understanding Repeat Antibiotic Prescribing in the Pandemic: Insights On Health Inequalities
- Trends in inequalities in avoidable hospitalisations across the COVID-19 pandemic
- Prescribing of Lidocaine Plasters
- The safety of antivirals and neutralising monoclonal antibodies used in prehospital treatment of Covid-19
- Investigating weight gain and obesity with OpenSAFELY
On OpenPrescribing we turn English NHS prescribing data into live, interactive tools for everyone to use. This includes a dashboard for every single practice in England on the “do not prescribe” list that was issued by NHS England in 2017. One of the items on this list is lidocaine plasters which was also “banned” by the Irish health service in 2017. We have teamed up with a group of researchers in Ireland to conduct an analysis of what happened in both countries and compare the outcomes of the different policy approaches used to restrict lidocaine patch prescribing by GPs.
The full academic manuscript is out now in the British Journal of Clinical Pharmacology. In our latest guest blog, Molly Mattsson from RCSI outlines what we found!
Our team at RCSI University of Medicine and Health Sciences in Dublin, together with the Bennett Institute for Applied Data Science, used OpenPrescribing to conduct our recent HRB-funded study on interventions targeting lidocaine plaster prescribing.
Why did we do this study?
Both the National Health Service (NHS) in England and the Health Service Executive (HSE) in Ireland identified prescribing of lidocaine 5% medicated plasters as a target for prescribing reduction measures1 2. This medicinal product’s licensed indication is for the treatment of post-herpetic neuralgia only. However, it had been prescribed and dispensed in volumes exceeding the likely prevalence of this condition, suggesting there was off-label use. Both countries introduced measures aiming to reduce lidocaine prescribing in 2017, but took quite different approaches, thus creating the opportunity for a natural experiment. In Ireland, restrictions on reimbursement were implemented, where individual patient approval was needed for prescribing to be covered, while in England, updated guidance on items which should not be routinely prescribed in primary care, including lidocaine plasters, was published.
What did we do?
We conducted an interrupted time series study using general practice data. Interrupted time series analysis involves tracking a long-term period before and after a point of intervention to assess the intervention’s effects, in this case volume of lidocaine dispensings. For Ireland, we used monthly dispensing data (2015-2019) from the General Medical Services scheme (which is means-tested, covering medicines for people based on their income and age). We requested this data from the HSE as it is not openly available. For England, we used openly available data from OpenPrescribing, thus covering all NHS patients. The outcomes of interest were the rate of dispensings, quantity, and costs of lidocaine plasters per 1,000 GMS/NHS population, and time series analysis was used to model level (immediate effect of the intervention) and trend (effect of the intervention on the direction of dispensing volumes) changes from the first full month of the policy/guidance change.
What did we find?
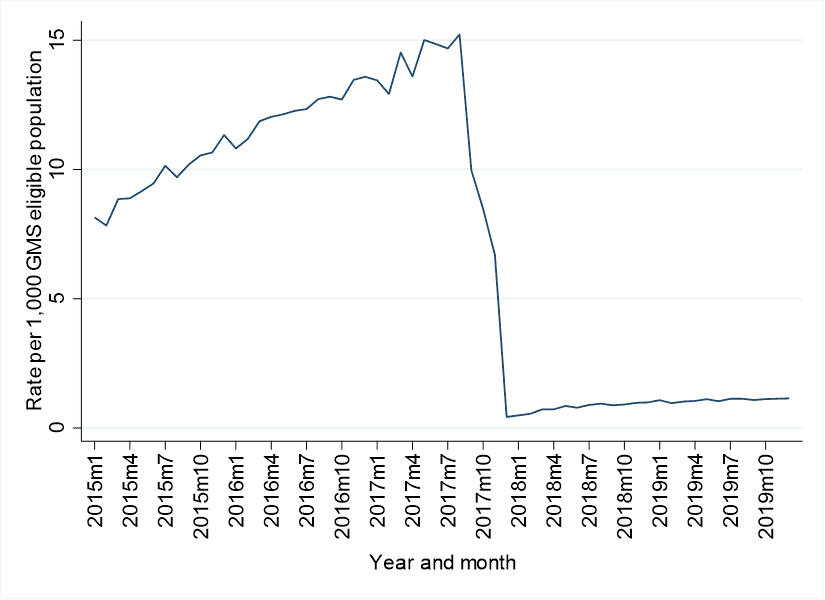
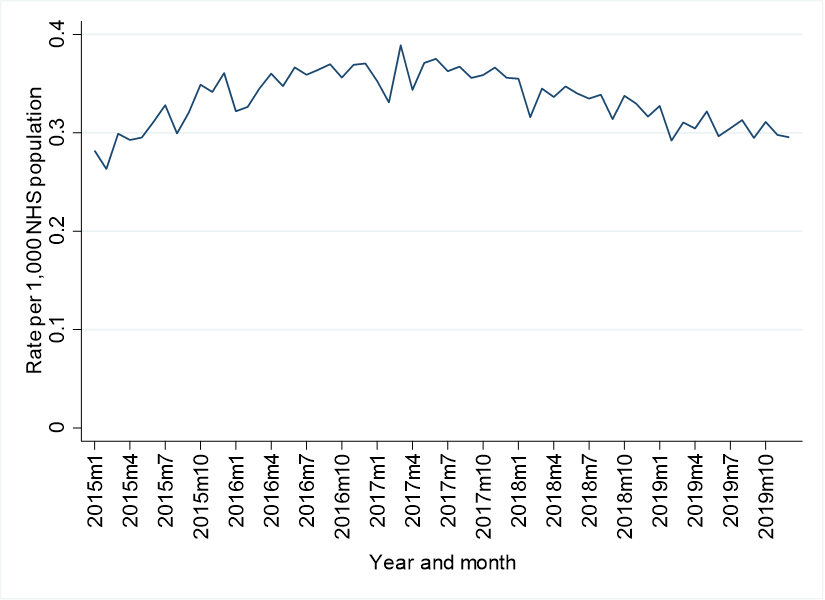
In Ireland, there was a substantial decrease of 97.3% in lidocaine plaster dispensings following the introduction of the individual patient approval system for reimbursement. In England, a very different pattern was identified. There was a small immediate decrease in prescribing the month after the guidance was introduced (a 5.8% reduction), followed by a gradual downward trend in the months after. As can be seen in the below figure, baseline rates of use were far higher (15 dispensings per 1,000 population versus 0.4) in Ireland compared to England, and even after a huge reduction, rates were still higher in Ireland post-interventions. We can’t be certain of the reasons for this disparity, or what the appropriate level of prescribing is but we think our study can help inform policymakers in understanding what types of policy interventions are likely to succeed in reducing prescribing. More work is needed, and is currently in progress, to understand the potential adverse consequences of these prescribing reduction strategies.
Lidocaine patches dispensings per 1,000 eligible population for Ireland (top) and England (bottom)
Conclusions
We found that in the context of much higher prescribing rates to start with, reimbursement restriction in Ireland was followed by a much greater reduction in use of lidocaine plasters than the introduction of guidance in England. This research relied on a data request to the HSE in Ireland. While we were grateful for their support of this study, making prescribing data openly available in Ireland would allow for routine evaluation of drug utilisation and facilitate more cross-country comparative analysis as we have proposed with OpenPrescribing.yourcountry.
-
Lidocaine 700 mg medicated plaster (Versatis®) - Prescribing and Cost Guidance (Medicines Management Programme) (2021). https://www.hse.ie/eng/services/publications/clinical-strategy-and-programmes/lidocaine-versatis-prescribing-cost-summary.pdf ↩︎
-
Items which should not be routinely prescribed in primary care: Guidance for CCGs (NHS England) (2019). https://www.england.nhs.uk/medicines-2/items-which-should-not-be-routinely-prescribed/ ↩︎