Zuclopenthixol Acetate: a new kind of measure on OpenPrescribing
- Posted:
- Written by:
- Categories:

Zuclopenthixol is an antipsychotic used for schizophrenia and other psychoses. In the UK, there are two injectable forms of zuclopenthixol:
- zuclopenthixol acetate : a short acting treatment used for acute episodes
- zuclopenthixol decanoate : a long acting treatment used for maintenance
This week we launched a new measure to support a new type of alert to identify any prescriptions of zuclopenthixol acetate for further investigation. It is not recommended to be prescribed in primary care, so prescriptions may have been prescribed in error.
What are the safety issues with injectable zuclopenthixol?
The BNF has a safety warning about both medicines advising that zuclopenthixol acetate preparations are used only in hospitals in acute episodes as it is a relatively short acting antipsychotic, while zuclopenthixol decanoate preparations are used in primary care to support long term maintenance. Furthermore, patients must be closely monitored in the period after the zuclopenthixol acetate injection is administered, which is best done in a hospital setting.
This video from the East London Foundation Trusts Medication safety team discusses some of the broader clinical and safety aspects related to zuclopenthixol acetate, and highlights similarities in the product packs.
In England last year 30,398 items for zuclopenthixol decanoate and 61 items for zuclopenthixol acetate were prescribed by general practices. Since the acetate form is only recommended in hospital, we think that it is likely that some of these items may have been prescribed in error, perhaps due to the similarity in names.
Is all prescribing of zuclopenthixol acetate in general practice unsafe?
Although the BNF recommends that prescribing of zuclopenthixol acetate should be restricted to hospitals, there may be good reasons in individual circumstances why it might need to be prescribed. We have built this new alert as a prototype to allow organisations to identify unusual prescribing which might require further investigation to determine its clinical appropriateness.
What is our new measure and alert?
We have developed a measure of the number of items for zuclopenthixol acetate as a proportion of all injectable zuclopenthixol items. Due to the low numbers, any level of prescribing in an organisation will immediately appear as an outlier (figure 1) and trigger an alert in our monthly practice or CCG email subscriptions (you can sign up for these via your organisation’s dashboard). As the numbers are so “standard measures” dashboard but it will appear in the safety and mental health dashboards, and for most organisations prescribing will be zero. You can read more about our range of clinical dashboards on this blog.
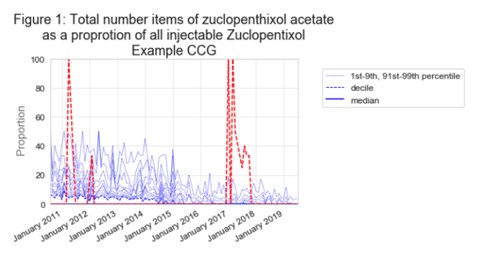
Excitingly, we have recently received funding from NIHR to produce a series of new tools that can systematically identify medicines and/or GP practices with unusual patterns of prescribing. This is the first prototype of a potential tool so we would really appreciate feedback about the measure or alerts to support the development of a range of actionable insights and useful tools for coalface clinicians. We will be advertising for a new researcher very soon to support this work, so please get in touch if you would like to work with our small but mighty team of software engineers, academics and clinicians.
At the Bennett Institute we pride ourselves on developing our tools swiftly and iterating them based on the needs of our users. We believe that the most useful tools are built by listening to our users and pooling their stories with the knowledge and skills of our small mixed team of clinicians, academics, and software engineers. Please get in touch and tell us what you think of this new measure supporting new types of alerts for and what improvements you would like to see at feedback@openprescribing.net.


