
- Posted
- Categories
-
- OpenSAFELY
OpenSAFELY at the National Patient Data Day in Leeds
On the 24th June 2025, OpenSAFELY had the privilege of running a booth at the National Patient Data Day in Leeds!
Latest news and views from around the Bennett Institute

On the 24th June 2025, OpenSAFELY had the privilege of running a booth at the National Patient Data Day in Leeds!

The Bennett Institute is hosting two days of talks and workshops on medicines, NHS data, and open research.
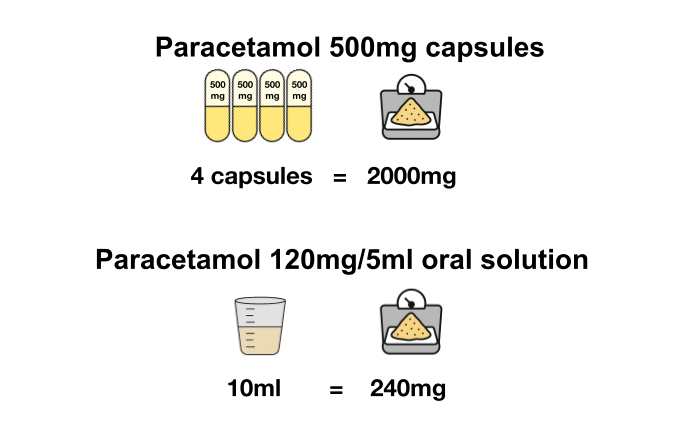
What is ingredient quantity, how can it be calculated from the quantity reported in the Secondary Care Medicines Data, and why is it useful?

In 2019, we pitched a national tool to track local formularies. Now the NHS is doing just that! We’re sharing our plans and advice to help it succeed.

Brian MacKenna demonstrated OpenPrescribing Hospitals at the 2025 Clinical Pharmacy Congress last month. Here’s his thoughts on how it went.
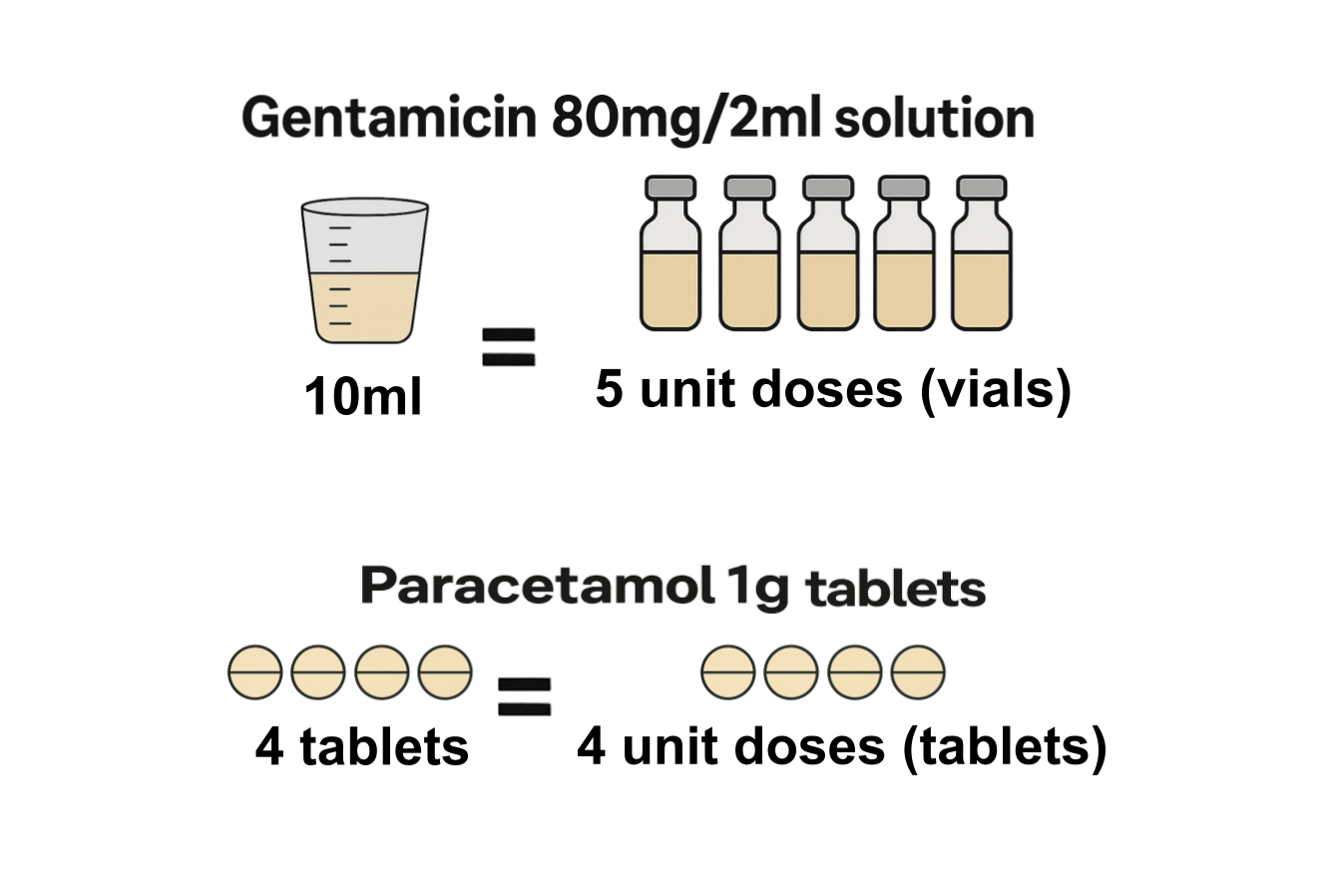
What are unit doses, how can they be calculated from the quantity reported in the Secondary Care Medicines Data, and why are they useful?

A summary of our discussion with NHS leaders on how Medicines Optimisation teams can leverage data science to enhance their strategic capability.

We’re bringing OpenSAFELY to education data - see the new OpenSAFELY Schools website to stay up to date!

Office hours, coding meetups and more! Come and talk to other OpenSAFELY users.
A look at how quantity is measured in the Secondary Care Medicines Data
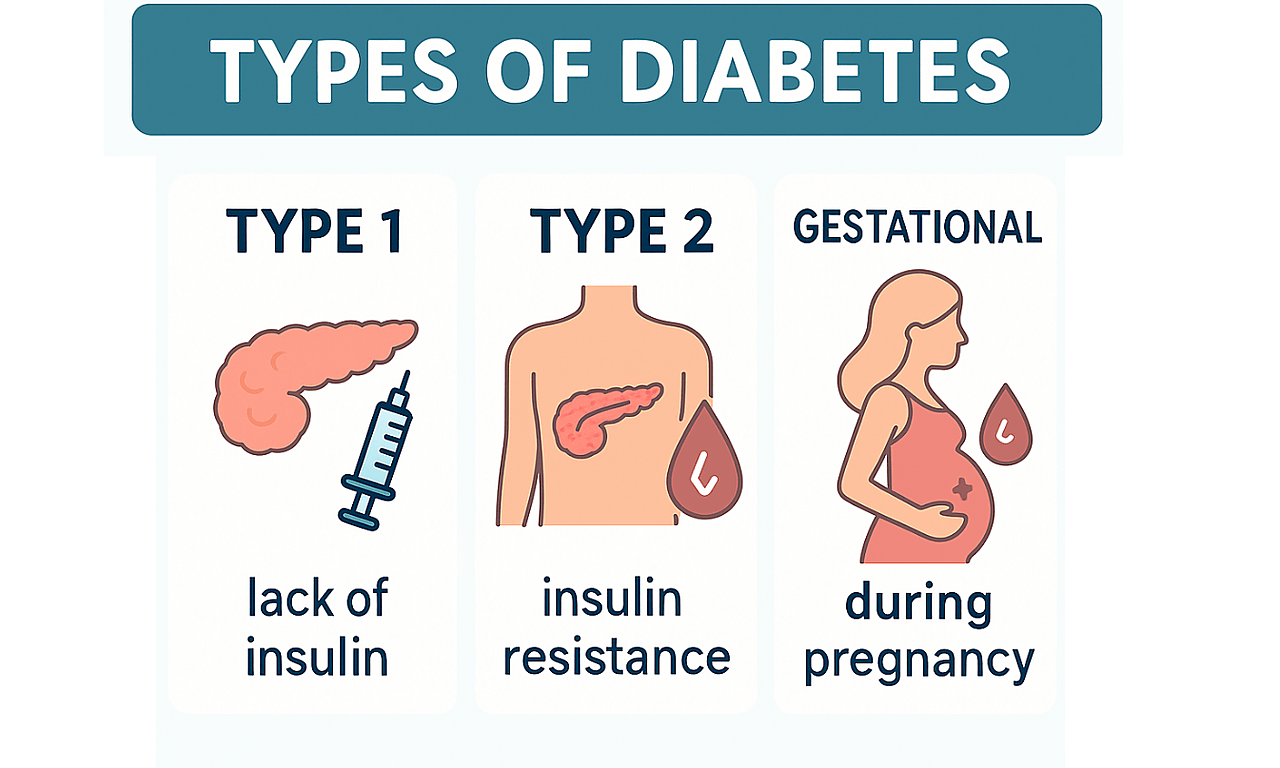
Together with collaborators in Bristol and at the Bennett Institute, we created a reusable action called ‘diabetes-algo’. Here is what it does.
Our tech team has been making improvements to OpenCodelists! What’s new?

In January 2025, Eli (our Research Software Advocate) sat down for an interview chat with Hannes Lowette and discussed all things OpenSAFELY.
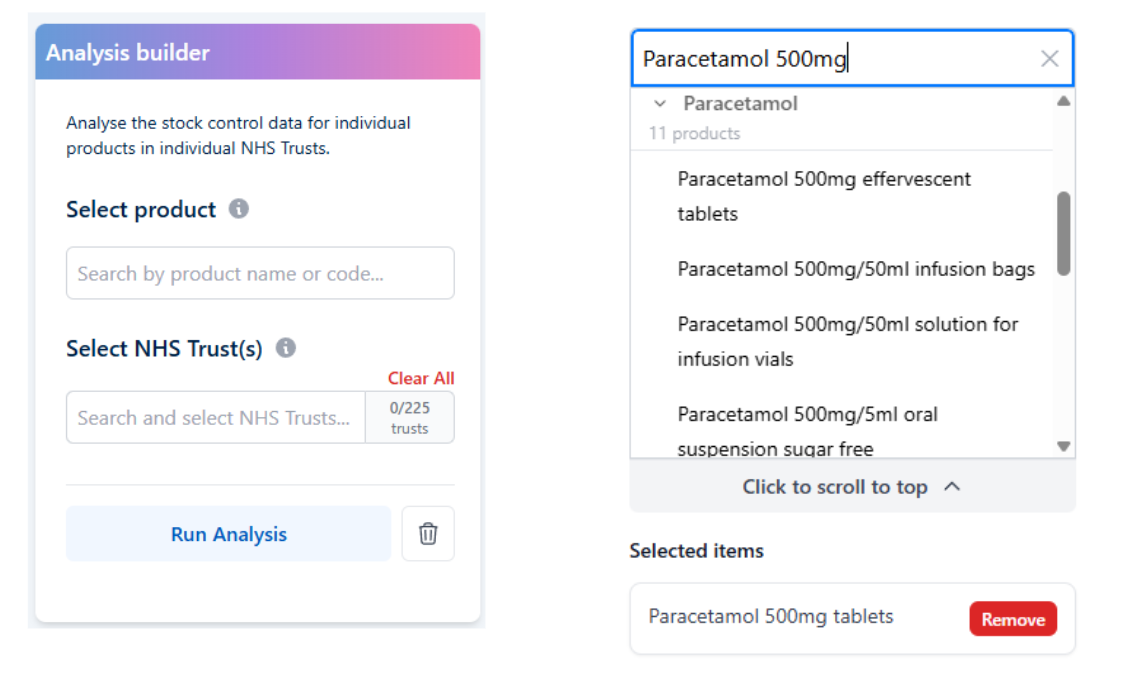
The new custom analysis feature on OpenPrescribing Hospitals.

We have vacancies for two senior software developers to work on OpenSAFELY and our other products

We’re looking for a product manager to come and work on OpenSAFELY with us.

How we can use OpenPrescribing Hospitals measures to look at use of low value medicines in hospitals.

How we highlight variation in hospital medicines usage in OpenPrescribing Hospitals Measures.